This blog post was originally published at NVIDIA’s website. It is reprinted here with the permission of NVIDIA.
Researchers studying cancer unveiled a new AI model that provides cellular-level mapping and visualizations of cancer cells, which scientists hope can shed light on how—and why—certain inter-cellular relationships triggers cancers to grow.
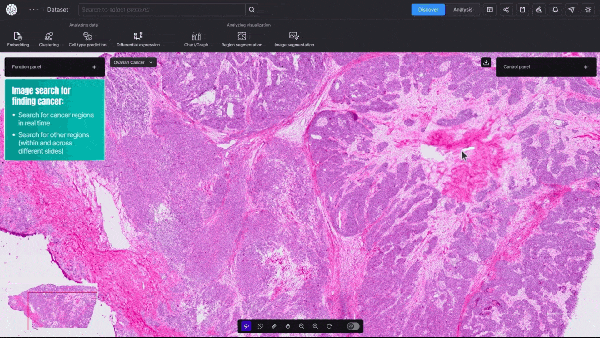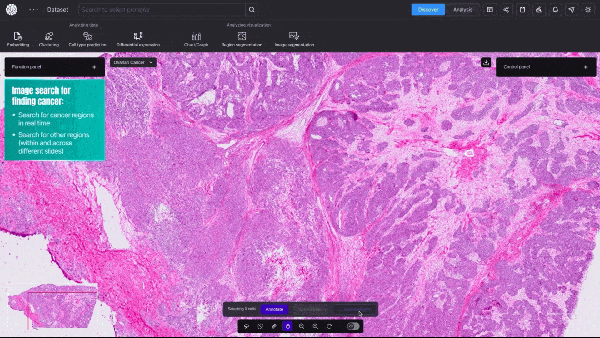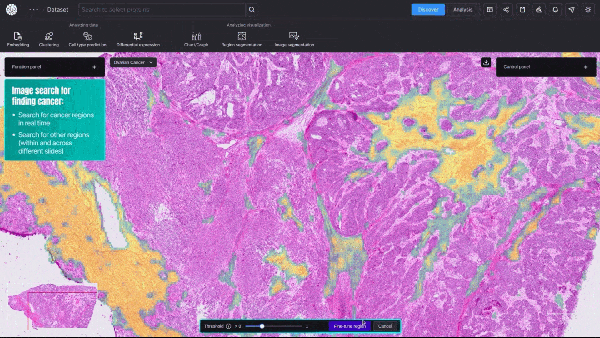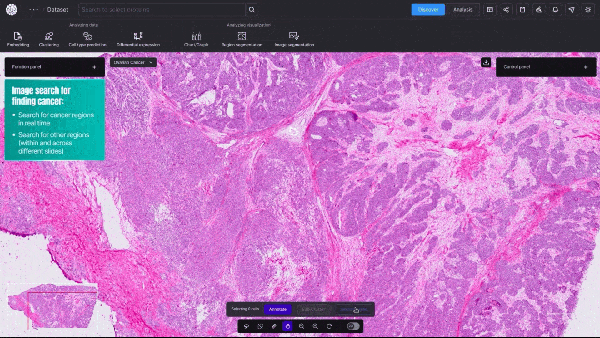
BioTuring, a San Diego-based startup, announced an AI model that can quickly create detailed visualizations of cancerous tumors—at single-cell resolution. This type of granular data shows a cell’s size, shape, which genes are activated, and, crucially, the relative spatial position of millions of different cells within a tissue sample.
Unlike traditional, far slower biological computation methods, the new model provides real-time, high-resolution insights into tumor dynamics and how cancerous and immune cells interact.
“There are around 30 trillion cells in the human body, and if you look at a large tumor biopsy, it has a few million cells,” said Son Pham, BioTuring’s CEO. “The analogy would be—imagine you’re analyzing satellite imagery with a very high resolution, trying to understand how a city works. What our model does, in the context of biology, is show you each house, what’s inside those houses, who’s talking to whom, and what they’re saying.”
“Similarly, our models let you see which cells are talking to which other cells, which groups are forming and talking amongst themselves, and what kind of relationships they’re forming—which can answer some of the most complex challenges in clinical oncological research.”
BioTuring, a member of the NVIDIA Inception program for startups, is pursuing its research in single-cell spatial omics, a subfield of biology which examines biological molecules—like messenger RNA and proteins—in their original spatial context in tissue.
Video 1. Demo of BioTuring’s SpatialX deep learning platform for unified multi-technology spatial data analysis.
To create its high-resolution mapping, or “disease cell atlases”, including of ovarian cancer cells, the team used NVIDIA H100 Tensor Core GPUs, and NVIDIA cuBLAS and NVIDIA cuSPARSE libararies to accelerate matrix operations in optimized analyses like the more traditional machine learning algorithm, the Weighted Gene Co-expression Network Analysis and CellChat.
Knowing how cancer cells develop and metastasize within a human body—and specifically, within a microenvironment within an organ—could improve screening methods for early cancer detection. Additionally, researchers can use the model’s cellular insights to better understand tumor heterogeneity—or cancerous tumors in the same patient with cells that materially differ from one another.
The new model’s enhanced visual granularity means researchers and drug developers have a far better chance at discovering molecular markers that can more accurately target cancerous cells.
The model can see, for instance, how a person’s killer T cells—humans’ disease-fighting cells—can change shape to engage cancers. By knowing how a person’s immune system morphs to fight a specific cancer, a drug developer could create synthetic therapies that support the patient’s immune system.
“We’re helping uncover biological discoveries that researchers can use to drive therapeutic strategies,” said Rujuta Narurkar, BioTuring’s COO. “Understanding the tumor’s microenvironment through various stages will help map the trajectory of cancer and potentially reveal the source of cancer itself. This new level of cancer tissue resolution has never been possible before. But new technologies are now bringing it within reach.”
Related resources
- GTC session: Accelerate Cancer Research With AI-Driven Multimodal Data Integration
- GTC session: An AI-Driven System for Large-Scale Digital Pathology Annotation and Characterization
- GTC session: Bridging Medical Gaps from Malaria to Cancer: On-Device AI Diagnostic Solutions Powered by NVIDIA Jetson
- NGC Containers: VISTA-3D NIM
- NGC Containers: MATLAB
- SDK: MONAI Cloud API
Elias Wolfberg
Executive Communications Team Member, NVIDIA